Can I join the team?
We are always looking to bring new members into the lab. This is how we stay young. If you want to work with us please see information in the Join Us page and send us a note. We prefer this to emailing directly.
Just a heads up – we sometimes get a little overwhelmed with messages, but we try our best to respond to everyone.
We want to hear from you!
Are you enrolling patients in clinical trials?
Not yet. But if you or someone you love would be interested in a biomaterials-based approach as part of the road to recovery, please consider leaving a post on our Patient Connection page. Your stories and support keep us motivated to find new solutions, and they add urgency to push toward clinical trials. By sharing an experience, you not only remind others that they are not alone, but you also encourage the rest of the scientific community to join us in finding solutions for the treatment of stroke and non-healing wounds.
For up-to-date milestone announcements, please follow us on Twitter.
Does your material work in humans?
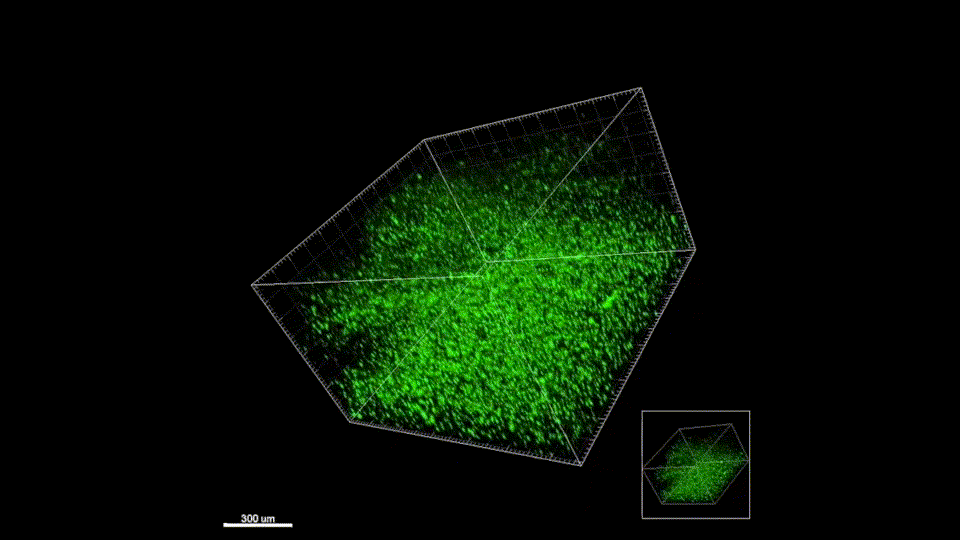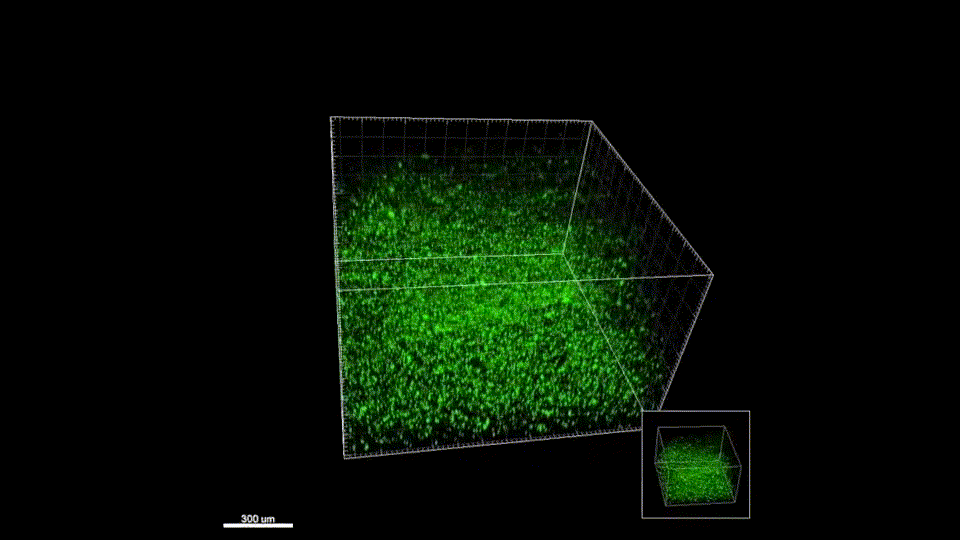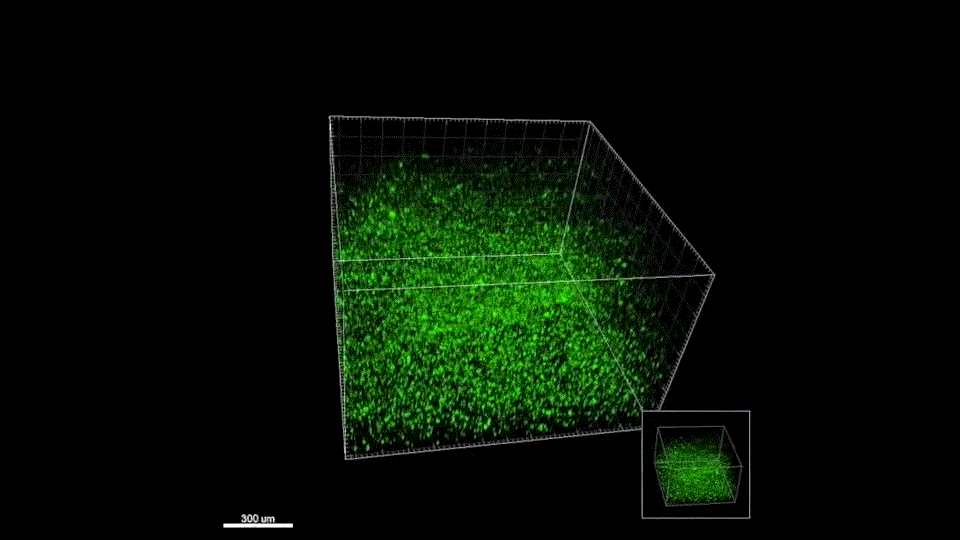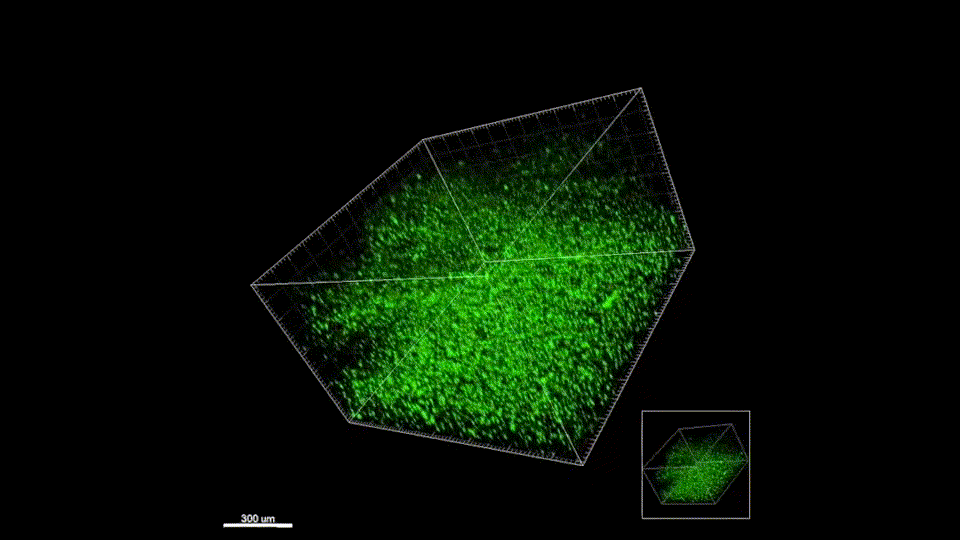
Our materials have not yet been tested in humans. The in vivo results presented on our Research page are gathered from murine (mouse) models. We are working to perfect the efficacy and safety of our materials before entering clinical trials. Follow us on Twitter where we post milestone announcements.
What is your approach to reduce physical disability after stroke?
We are developing biomaterials that are designed to be injected into the stroke cavity of the brain in order to promote neuronal growth where tissue has been lost. By encouraging tissue regrowth, we aim to restore function and reverse disability.
Physical disability after stroke is the result of brain tissue that was permanently damaged while the brain was deprived of oxygen. Current stroke treatments must be administered within hours after the attack and aim to minimize neuronal death (think damage control), which in turn minimizes the severity of disability. In contrast to this standard of care, our material will offer patients an additional therapeutic option that can be administered days to months after the stroke with the goal of catching the brain during the healing process and encouraging it to regenerate dead tissue instead of filling the space with a non-functional scar. In this way, our material attempts to recover brain function and reduce physical disability. Read more on our Research page.
What is your approach to promote skin wound healing?
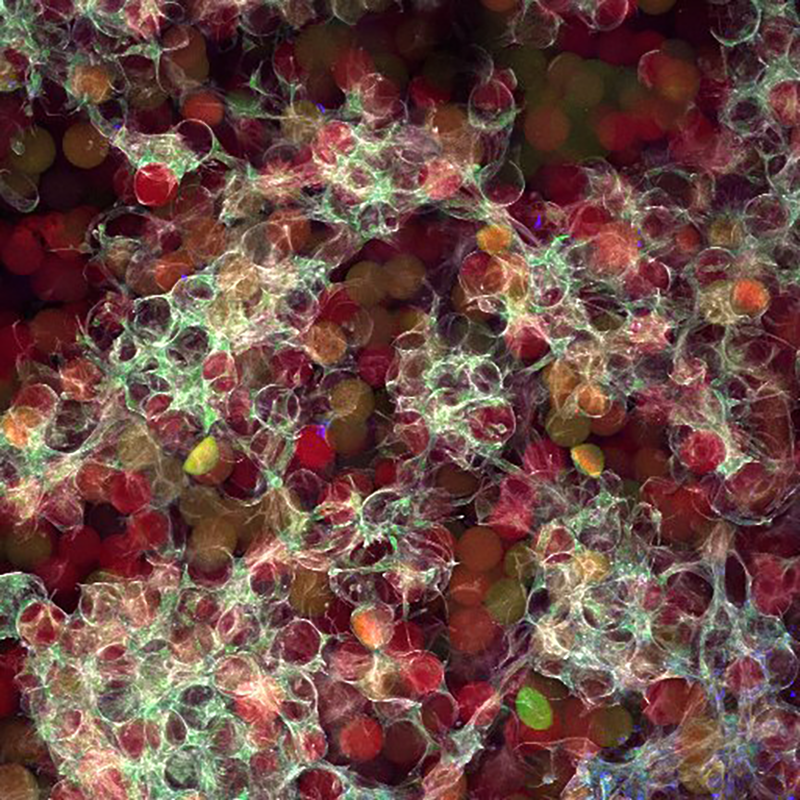
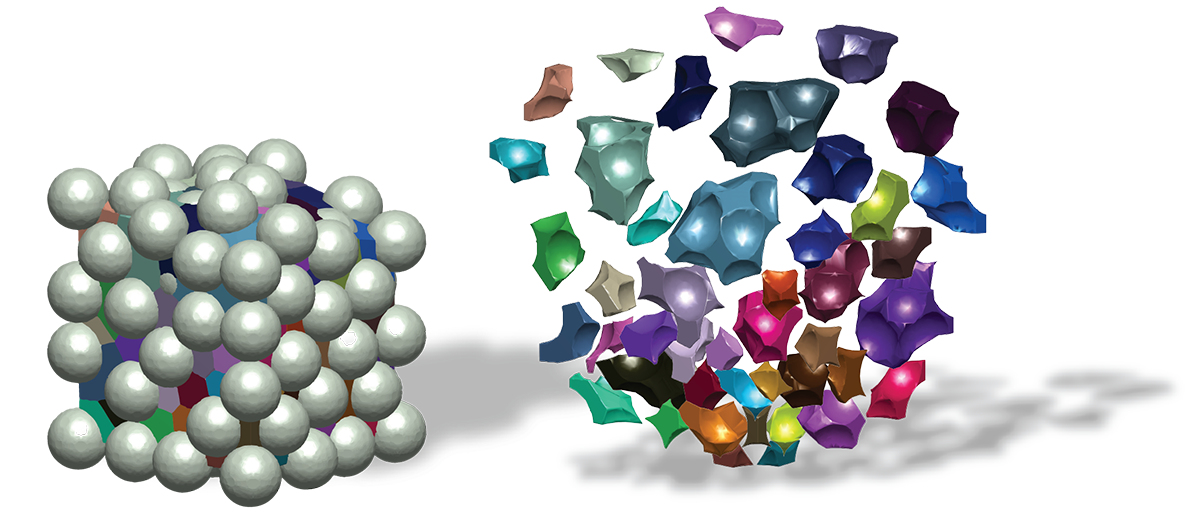
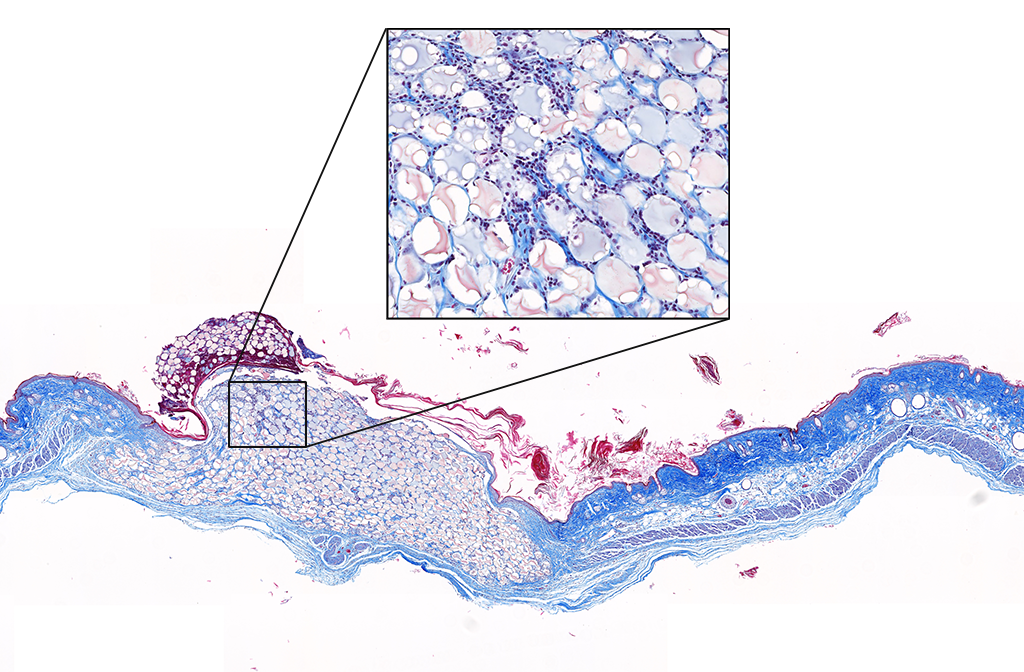
We are engineering biomaterial gels that can be delivered directly into skin wound beds to behave as tissue mimics that speed up the healing process and improve the overall outcome of skin quality. In cases where patients have wounds that never fully heal, our materials are designed to calm inflammation in order to allow the body to progress to the later stages of wound healing. The secret to our madness is in the chemical composition and microarchitecture of our gels that are engineered to influence the cellular microenvironment of the wound. We go into more detail on our Research page.
What are your biomaterials made out of?
The majority of our materials are made from either hyaluronic acid (HA) or polyethylene glycol (PEG). HA a naturally-derived polymer found in abundance throughout our bodies (you may have noticed this ingredient in a lot of cosmetic products aimed at rejuvenating the skin). PEG, on the other hand, is a synthetically-derived polymer that has been studied for years and has been used in a wide range of medical products because of how safe it is (it gets the FDA stamp of approval ✅). After choosing a base material, we then perform a number of chemical modifications to get the polymers up to speed for our wound healing applications. But of course, we can never stop exploring, and our materials are not limited to HA and PEG.
Are you accepting new students or postdocs into your lab?
Yes, we are always looking for talented, self-driven, inspired students / postdocs to work with us. Please visit our Contact page for ways to reach out and / or apply.
Do you collaborate with other labs?
Yes, science and progress rely on collaborations! We don’t have a consolidated list of all of our collaborations; however, our Publications page may give a clue. If you are interested in learning how to make our materials or in using our materials for new applications, please reach out using our Contact page.
Is your technology patented?
Yes, we patent our technology whenever possible. We have 9 issued patents, 14 applications, and several more that have been disclosed. The most recent patents cover our MAP scaffold technology and our D-peptide technology, but you can find all of our patents on our Publications page.
What are your funding sources?
The Segura lab has been very fortunate to be funded through a variety of sources since its early years. More recently in 2020, Professor Segura received three NIH R01 grants totaling more than $6 million to use toward developing regenerative biomaterials for brain and skin.
While we’re on the subject, we’d like to take this opportunity to thank all of our sponsors. A special thanks goes to our biggest supporters: the National Science Foundation, the National Institutes of Health (NIH), and the American Heart Association.
What's up with this swanky website?
This website exists because Professor Segura was awesome enough to let Lindsay go wild with her vision for a fun and feminine lab website – who says science has to be sterile and unisex? The design was inspired by her favorite websites, and all of the content was generated in-house – meaning anyone can do it too! Cartoons and animated gifs were created in Procreate – cinemagraphs were created in Photoshop – videos were edited in iMovie – and for pictures, if it wasn’t taken on a microscope, it was pretty much taken with an iPhone.
To bring her vision to life, the Segura lab partnered with the incredible team at Citrus Studios in Los Angeles, CA. Reach out! They’re the friendliest group of web developers you’ll meet. And for Youtube videos where Tatiana Explains, we’re leaving the editing to our friend Nidal in Los Angeles.