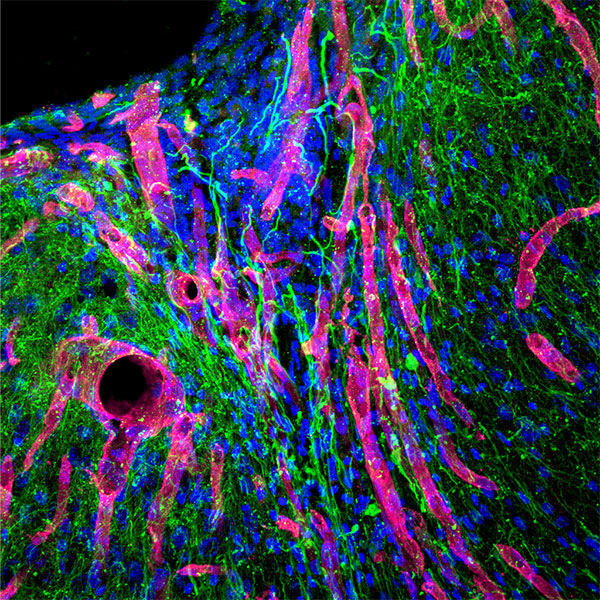
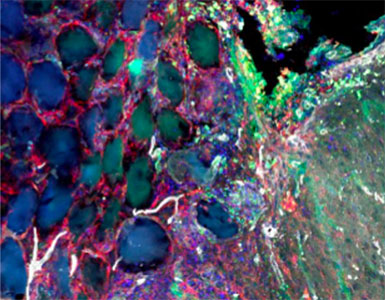
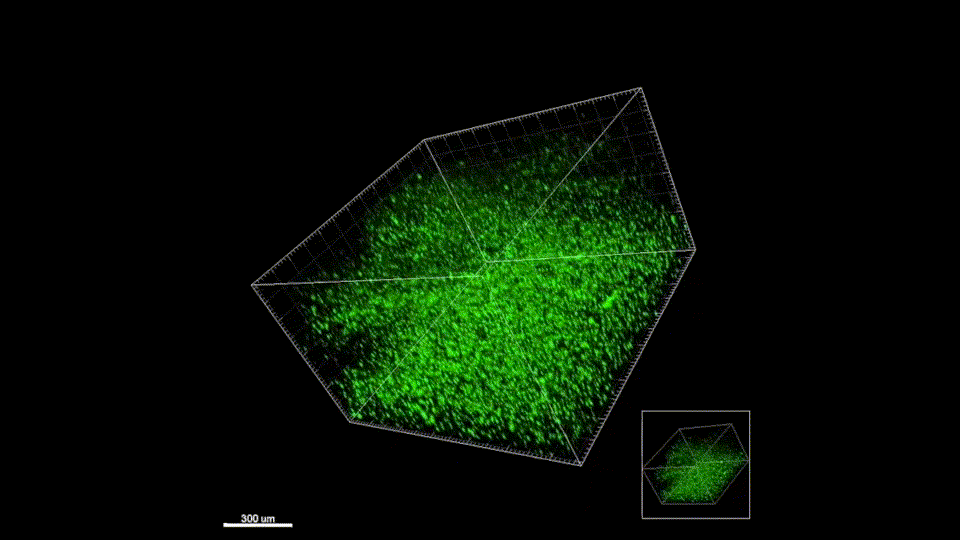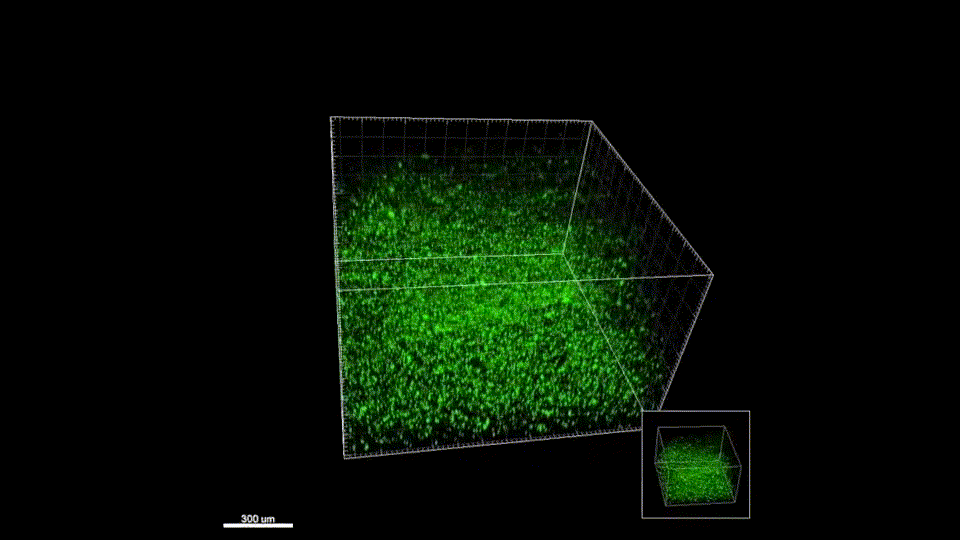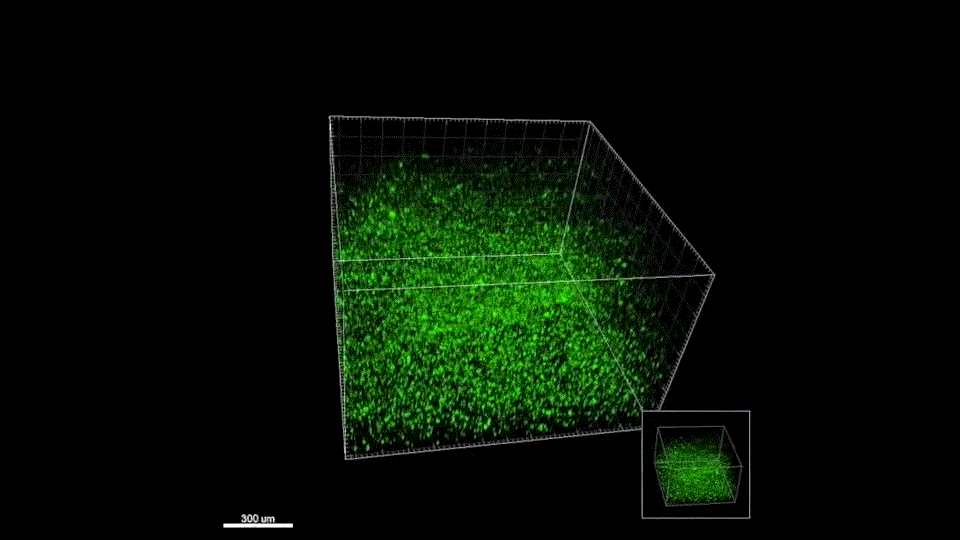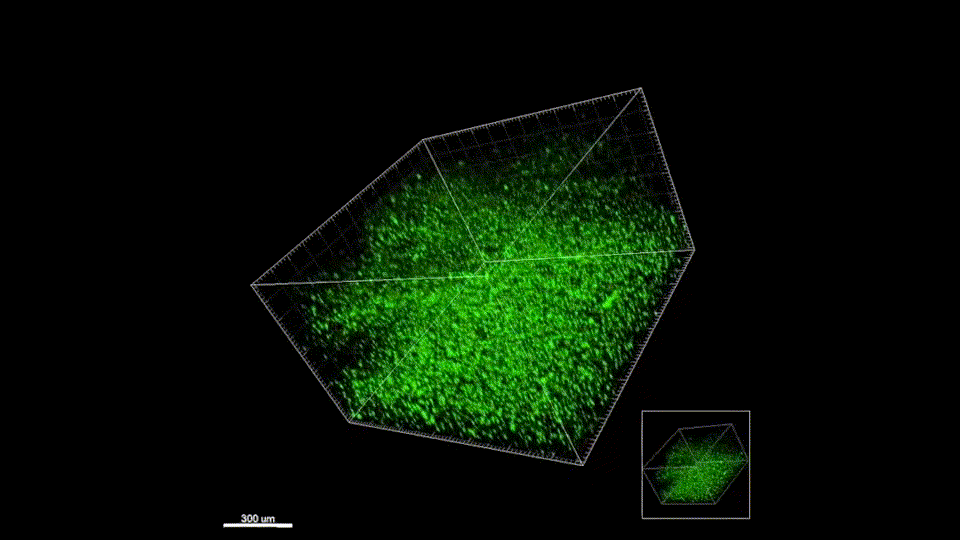
Response from: Professor Tatiana Segura
[from Lindsay Riley, PhD] Peyronies disease is indeed devastating, and this is a very meaningful avenue to pursue regarding biomaterials to minimize scarring. To date, the lab has not investigated prior art in the space of Peyronies treatment; however, when we have the time, we will look into whether models exist for recreating and studying the disease. Hydrogel biomaterials may be the key to lessen scarring for those who suffer from Peyronies. Thank you for bringing the importance of Peyronies research to our attention. We hope others in the field are inspired by your message.We are deeply motivated and affected by the stories shared by real patients and families.
We welcome you to ask questions, share your thoughts, or introduce us to the individuals we are working to help.
- Tuesday 08/01/23 Max Europe
- Friday 04/14/23 Scott Wisconsin
Response from: Professor Tatiana Segura
Scott, your words of encouragement made our day. There are multiple students in my lab who are dedicated to developing translational technology for minimizing scarring, and it makes a difference for young scientists to hear first-hand that their work is valued and impactful. We wish you the best, and I hope to have exciting updates to share in the future. - Wednesday 02/15/23 Harsh India
Response from: Professor Tatiana Segura
Dear Harsh, I'm very saddened to hear that you suffered brain damage as a result of kernicterus. As a lab, we have never studied kernicterus, so we appreciate that you shared your story. Our biomaterial experiments are currently performed in mice using an induced-stroke model, so unfortunately, I have no information on how the material would fare in a different disease model. While we are currently focusing our efforts on studying stroke in the brain, we are always open to expanding our studies. This usually occurs through collaborations or if a new student joins the lab with an interest in a different disease state. Per our email exchange, we hope to hear from you regarding your interest in a masters! - Thursday 10/06/22 Chris Boston, MA
Response from: Professor Tatiana Segura
Hello Chris, thank you for your question. We are slowly getting to chronic stroke. As you know, chronic stroke is is harder to treat than acute stroke because the post-stroke reparative window is closed. So far, all of our testing has been in the sub-acute time period; however, we do believe we can engineer our MAP material to re-open this window. - Saturday 03/26/22 Lisa Piermont, NY
Response from: Professor Tatiana Segura
Lisa, thank you for sharing your daughter’s story. It’s incredible to hear about her neuroplasticity. We are not at the stage of clinical trials, so unfortunately, we don’t have an expected date. However, hearing about stroke in such a young patient who has shown signs of replasticity is immensely motivating to my lab and the scientific community. If you go onto the Publications page of our website and filter by ‘Topic > Stroke,’ you will be able to see our relevant publications. If you include ‘Type > Primary research’ in your filter, you will see our novel work in the field. Please don’t hesitate to reach out through our Contact Page if you have additional questions. - Saturday 02/19/22 Alan Harris Statesville, NC
Response from: Professor Tatiana Segura
Alan, you have shared an absolutely devastating story that has touched the hearts of everyone in our lab. I am shaken to hear of such an extreme outcome from stroke, and you have raised awareness for how serious the condition can be. We have not yet studied stroke in the basilar artery, but thank you for bringing this important site to our attention. Clinical trials are our goal, and your story serves as sincere motivation for pushing our technology in that direction. - Sunday 02/13/22 Joey Europe
Response from: Professor Tatiana Segura
Joey, thank you for sharing your story. It is so important for my team and me to learn about all of the different people we could help. So far, we have not worked on treating pre-existing scars but rather preventing scarring during initial healing. However, your story brings attention to this important problem, and I hope it inspires a solution. - Sunday 05/09/21 Rachel Smookler Charlotte, NC
Response from: Professor Tatiana Segura
Rachel, thank you for sharing your story. As you read, we are working on a biomaterial-based approaches to treat the disability caused by stroke. Although we have some promising results, we have only tested them in small animal models of stroke, so it would be some time before we can test them in a clinical trial. I am very moved that you found our research and found some hope in it. - Wednesday 05/05/21 Steve USA
Response from: Professor Tatiana Segura
Steve, thank you for sharing Derek's story. It is very crazy to me that we still have such a hard time closing up skin wounds. But it is true, some wounds just do not heal well or at all. For skin, our technology is much closer to the clinic. We have licensed our MAP hydrogel to Tempo Therapeutics in San Diego, and they are getting ready for FDA approval. Please keep checking our website and their website for information on a possible in-patient trial. We are continuing to improve the material as well to improve the strength of the tissue that gets deposited.