Advancing Wound Healing: from Surgical Technology to New and Improved Hydrogel Therapies
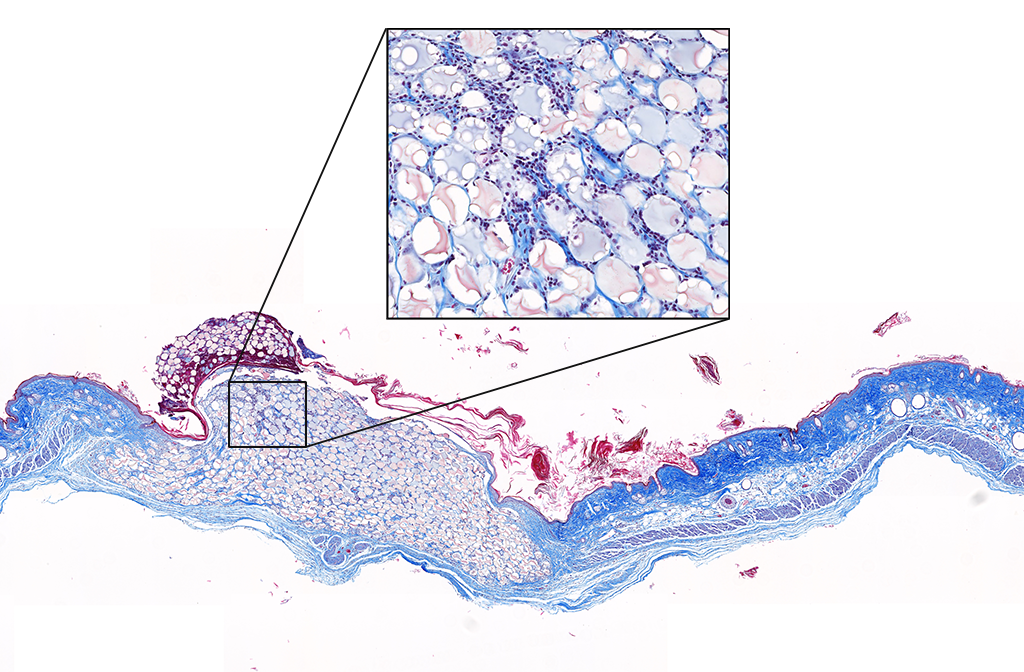
From Drew’s dissertation:
From Drew’s dissertation:

Read the full dissertation here: Advancing Wound Healing: from Surgical Technology to New and Improved Hydrogel Therapies
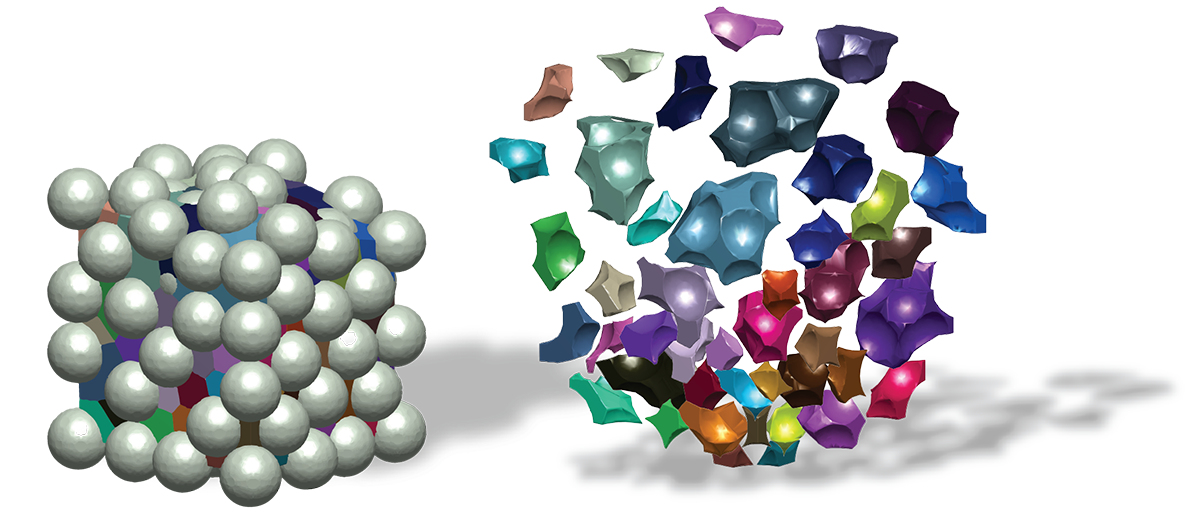
From Alex’s dissertation:
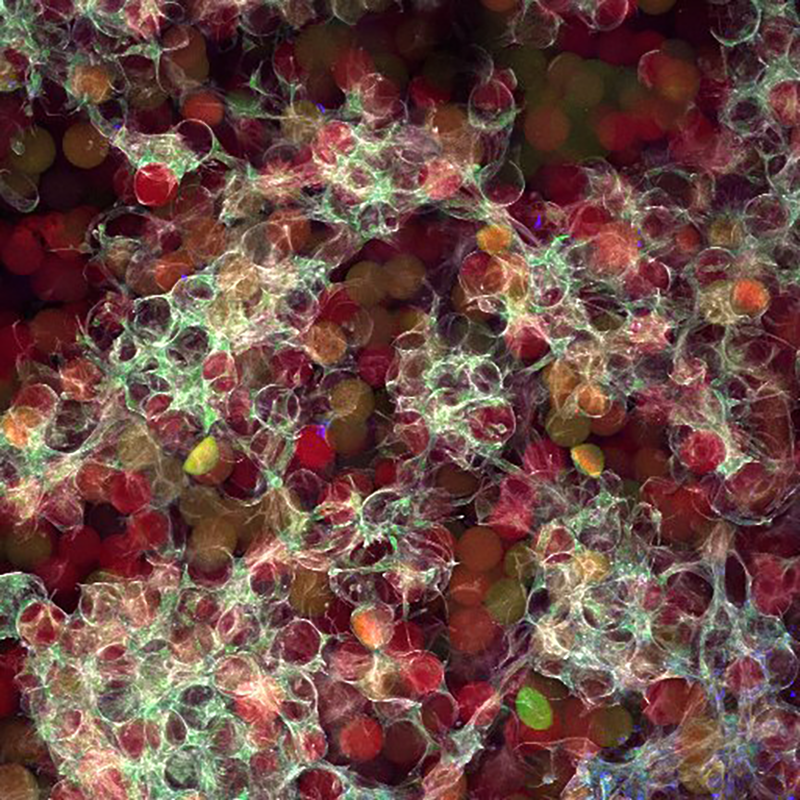
Read the full dissertation here: Engineering the microstructure and spatial bioactivity of granular biomaterials to guide vascular patterning
Straight from Evan’s dissertation abstract:

Straight from Kat’s dissertation abstract:

Straight from Yining’s dissertation abstract:

From Yasha’s lab diary:

From Andrea’s lab diary:

From Cara’s lab diary:

From Pablo’s lab diary:

Straight from Lindsay’s dissertation abstract:




From Sydney’s lab diary:

From Shangjing’s lab diary:

From Briana’s lab diary:



Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
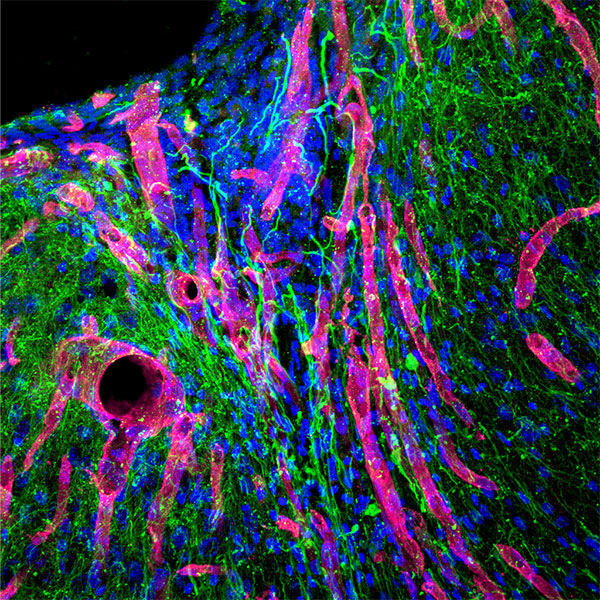
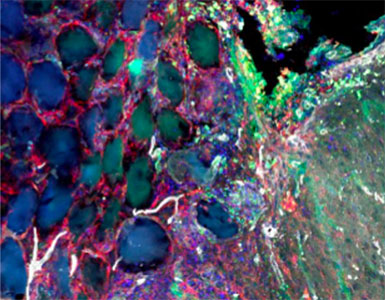
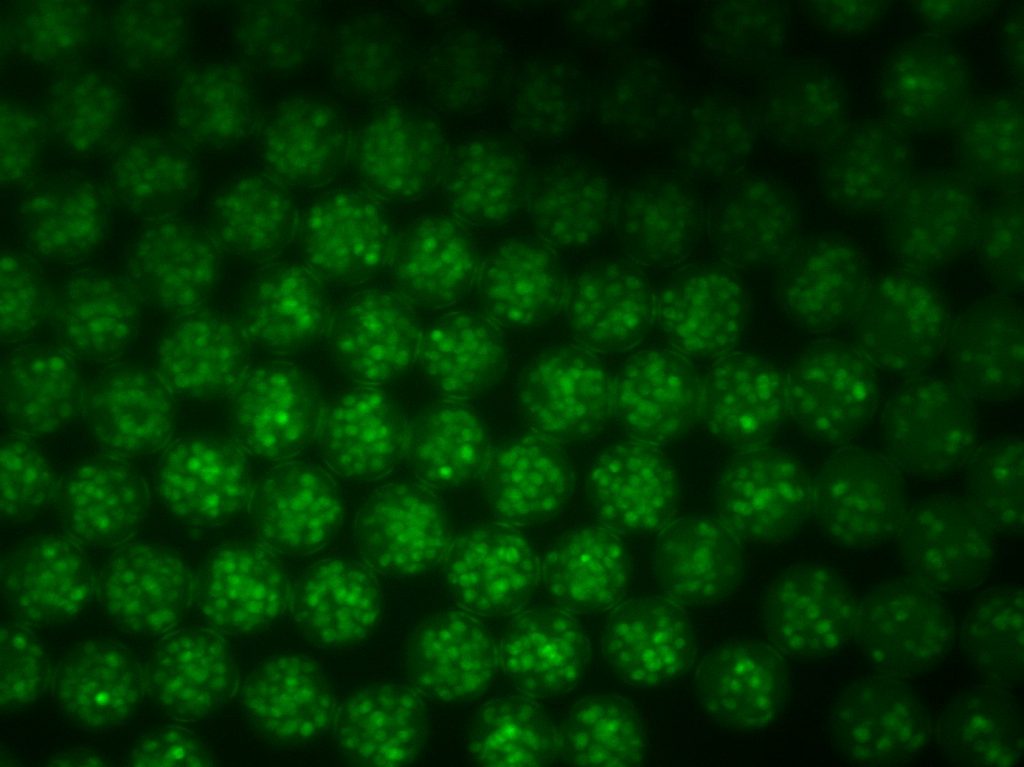
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

From Elle’s lab diary:

From Nhi’s lab diary:


From Aleja’s lab diary:

From Yunxin’s lab diary:

From Holly’s lab diary:








From Juhi’s lab diary:





From Kevin’s lab diary:










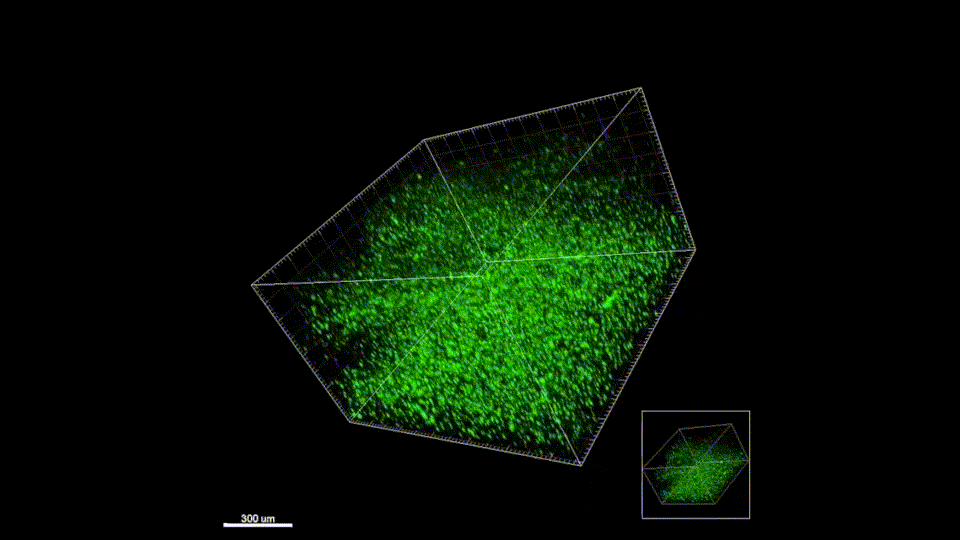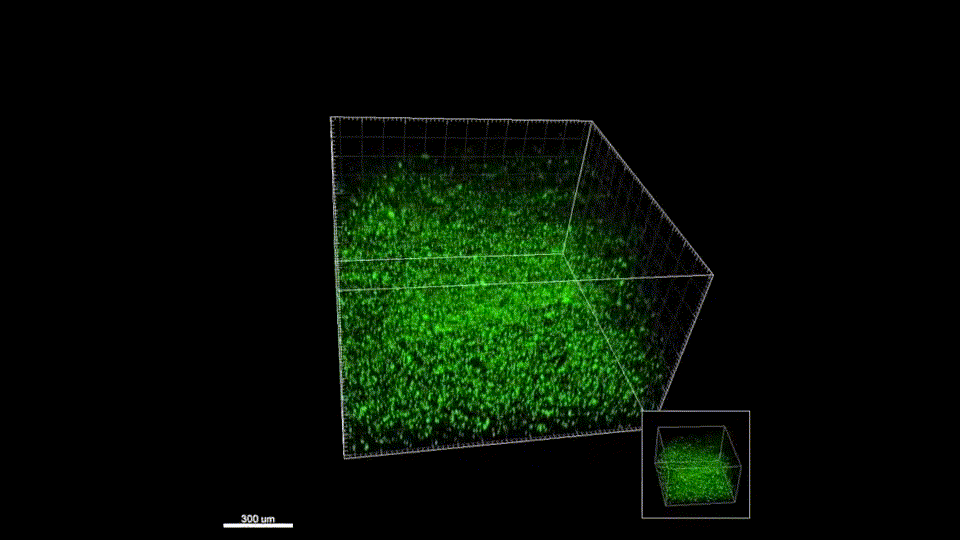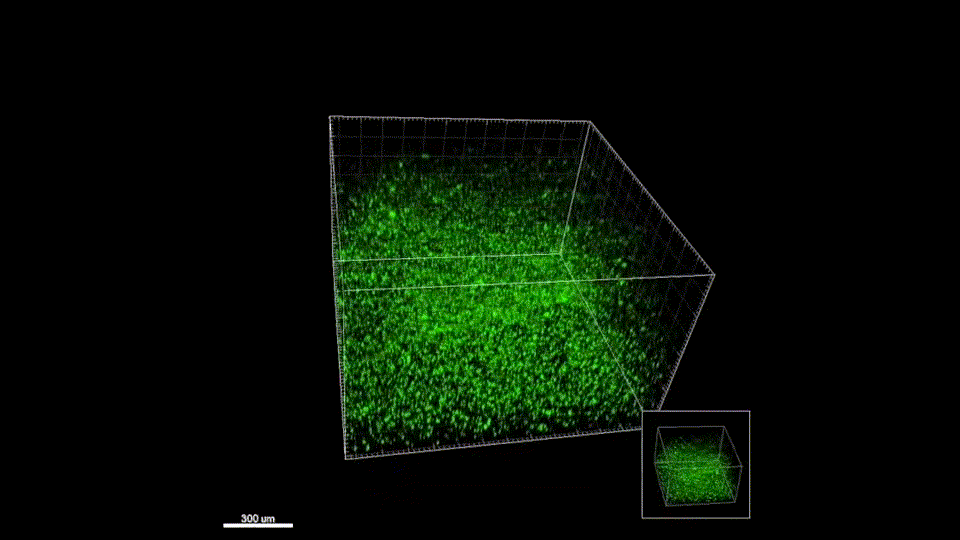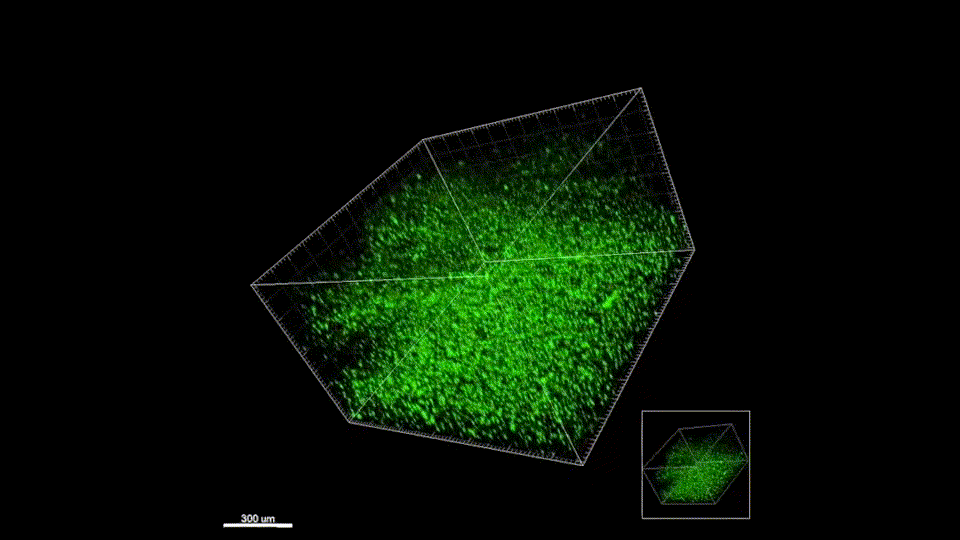

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.

Sept 2015 – June 2019
Elias Sideris
Clearview Healthcare Partners
Elias obtained his PhD from the Segura Lab in 2019 with a thesis titled “Hydrogel scaffolds and their influence on the brain.” He is now a consultant at Clearview Healthcare Partners based in San Francisco.
Mauris non tempor quam, et lacinia sapien. Mauris accumsan eros eget libero posuere vulputate. Etiam elit elit, elementum sed varius at, adipiscing vitae est. Sed nec felis pellentesque, lacinia dui sed, ultricies sapien. Pellentesque orci lectus, consectetur vel posuere posuere, rutrum eu ipsum. Aliquam eget odio sed ligula iaculis consequat at eget orci. Mauris molestie sit amet metus mattis varius. Donec sit amet ligula eget nisi sodales egestas. Aliquam interdum dolor aliquet do.
Mauris non tempor quam, et lacinia sapien. Mauris accumsan eros eget libero posuere vulputate. Etiam elit elit, elementum sed varius at, adipiscing vitae est. Sed nec felis pellentesque, lacinia dui sed, ultricies sapien. Pellentesque orci lectus, consectetur vel posuere posuere, rutrum eu ipsum. Aliquam eget odio sed ligula iaculis consequat at eget orci. Mauris molestie sit amet metus mattis varius. Donec sit amet ligula eget nisi sodales egestas. Aliquam interdum dolor aliquet do.