Altmetric Score
Dimensions
Citation
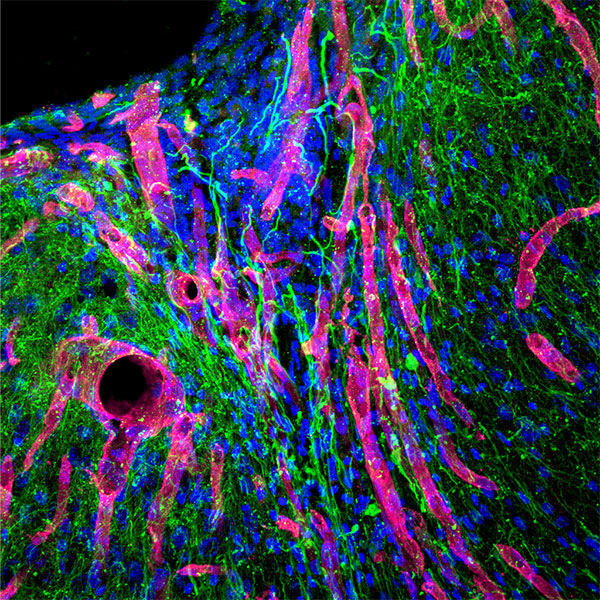
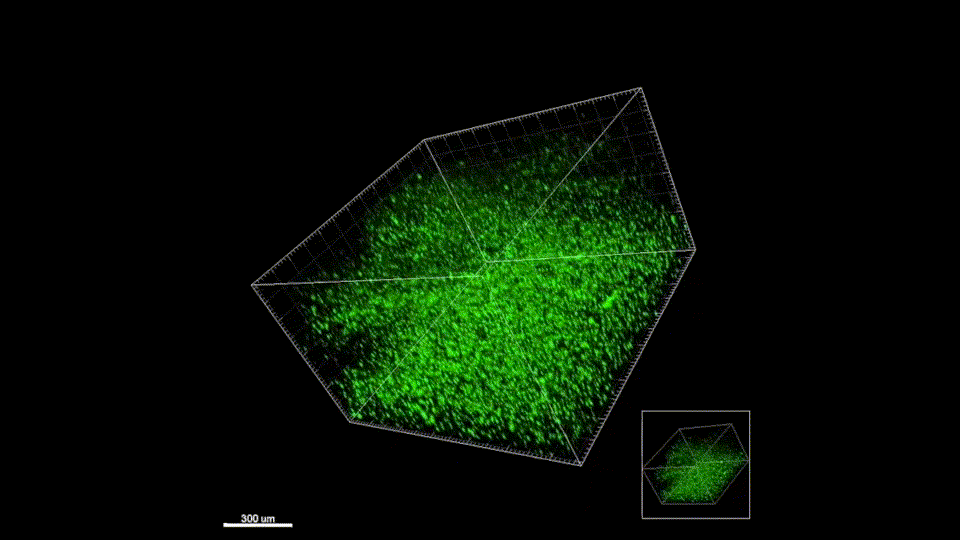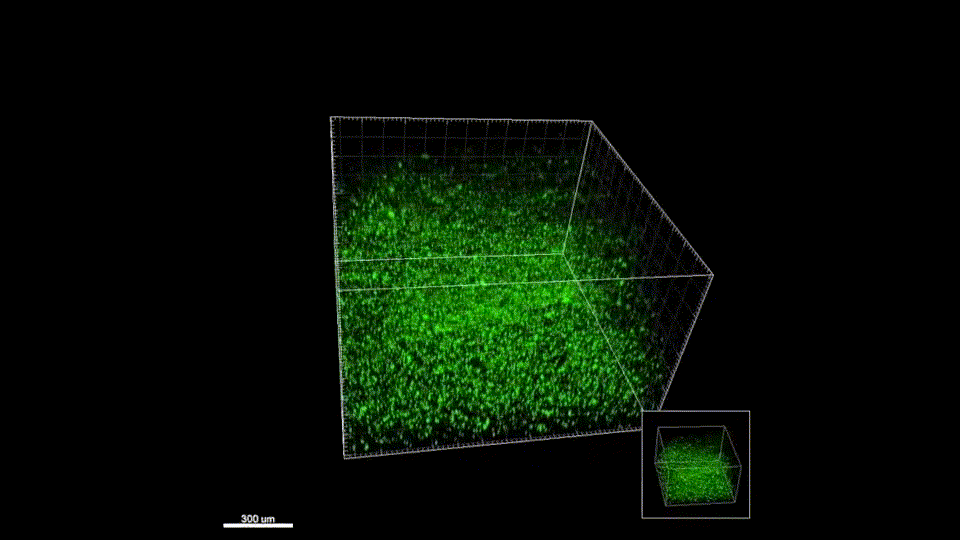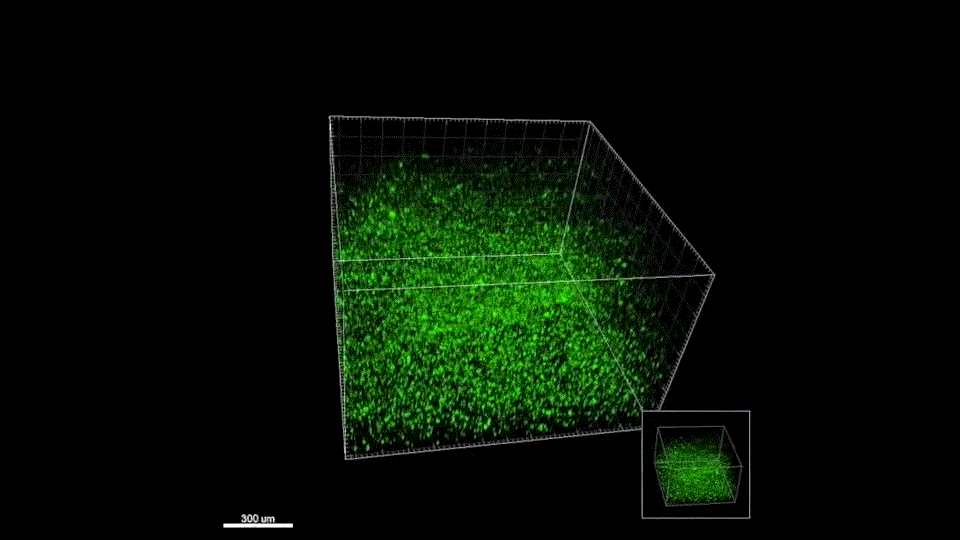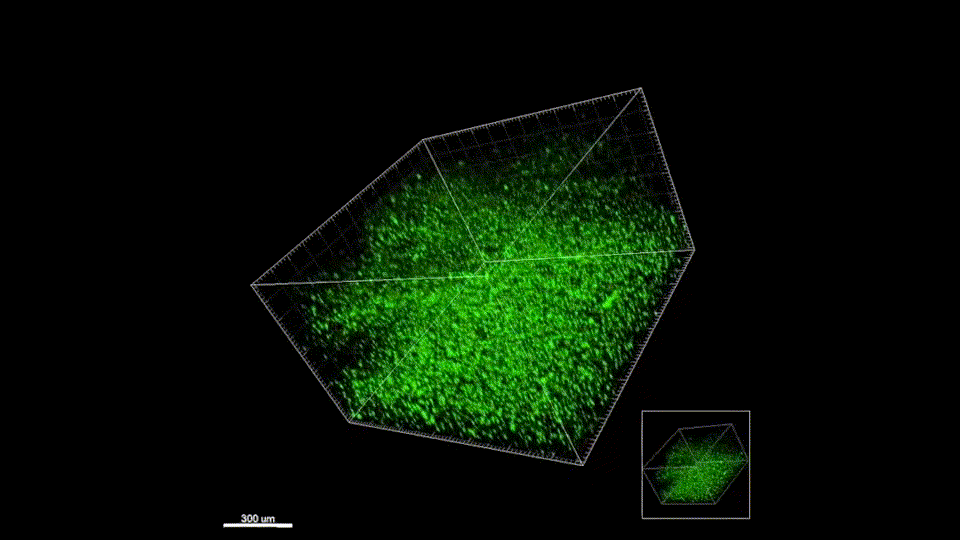
Stroke is the leading cause of disability and the fifth-leading cause of death in the United States. Approximately 87% of all strokes are ischemic strokes and are defined as the sudden blockage of a vessel supplying blood to the brain. Within minutes of the blockage, cells begin to die and result in irreparable tissue damage. Current therapeutic treatments focus on clot removal or lysis to allow for the reperfusion and prevent more severe brain damage. Although transient brain plasticity may salvage some of the damaged tissue over time, significant fractions of patients are left with neurological deficits that will never resolve. There is a lack of therapeutic options to treat neurological deficits caused by stroke, emphasizing the need to develop new strategies to treat this growing patient population. Injectable biomaterials are currently being designed to enhance brain plasticity and improve endogenous repair through the delivery of active agents or stem cells. One method to test these approaches is to utilize a rodent stroke model, inject the biomaterial into the stroke core, and assess repair. Knowing the precise location of the stroke core is imperative for the accurate treatment after stroke, therefore, a stroke model that results in a predictable stroke location is preferable to avoid the need for imaging prior to injection. The following protocol will cover how to induce a photothrombotic stroke, how to inject a hydrogel in a controlled and precise manner, and how to extract and cryosection the brain while keeping the biomaterial intact. In addition, we will highlight how these same hydrogel materials can be used for the co-delivery of stem cells. This protocol can be generalized to the use of other injectable biomaterials into the stroke core.